Clinical evaluation report medical device example
Clinical Evaluation for Medical Devices. prepare a clinical evaluation report including literature review and determine requirements for post-market clinical
Article explains the key steps to preparing a successful clinical evaluation report (CER) for submission of a technical file for medical device CE Marking.
A clinical evaluation is a required element of the conformity assessment process in the European Union (EU) for medical devices, and serves to substantiate
The clinical evaluation report and the clinical data Clinical evaluation of medical devices that of the device. The criteria used in the example below
Clinical Evaluation Reports: “Clinical evaluation of medical devices that are based on existing, For example, is the data from a
Clinical Evaluation Reports Now Required for All Medical Devices in Europe. There are changes sweeping European medical device regulations and these changes affect
The Impact of New CE Marking Rules Medical Devices Group

Clinical Evaluation of Medical Devices Qserve Group
25/01/2017 · In June 2016, the MEDDEV 2.7.1 rev 4, the European guidance on the clinical evaluation of medical devices (MD) was published. The issuance of this guidance
Requirements for clinical evaluation When a medical device is placed on the market, the manufacturer must have demonstrated the clinical evaluation Report
Generating clinical evaluation reports A guide to effectively analysing medical device safety and performance Hassan Achakri, PhD, Director, International Clinical

Study—Retrospective Evaluation Medical Devices—Post-Market Clinical Follow information from patients’ medical records via a structured case report
Webinar course provides a guideline on how to prepare a clinical evaluation for a Medical Device, considering the applicable laws and standard.
Public Health Europe in this new version of the SCCS Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation Flash report
Clinical Evaluation Report The Clinical Evaluation Report (CER) Clinical Evaluation Report Medical devices which have a high-risk classification or are not
Abstract Clinical evaluation is a structured ongoing procedure to collect, appraise and analyse clinical data pertaining to a medical device. The clinical data
Steps preceding a clinical evaluation Recently, a medical device company The clinical evaluation report is should also be attached to the report. An example
Evaluate the impact of regulatory changes on the medical device new devices with case study examples. a clinical evaluation report (CER) with medical
A Clinical Evaluation Report (CER) documents the conclusions of a clinical evaluation of your medical device. A CER consists of analyzed clinical data that was

clinical evaluation reports. ensure your medical devices comply with the clinical requirements of the medical devices directive: the eu me dical devices directive as
Preparing For Your Clinical Evaluation Report. the 10th Annual Q1 European Medical Device Clinical Research conference is a must-attend for forward-thinking
Template – Clinical Evaluation Report V03– 2017-0516. Letter-Template V01 – 2016-06-30. ISO 10993-1 Biological evaluation of medical devices – Part 1:
Clinical Evidence for IVD Medical Devices – Clinical Performance Studies for In conduct and report of a clinical Designs of IVD medical device clinical
Clinical evaluation process flow & checklists of HCL is an efficient way for products to comply with the MED DEV 2.7.1 Rev 4 and Medical device regulations (MDR
[Example] [4] Selection criteria Clinical Evaluation Reports are more important in Europe today tion reports for a large medical device company.
CLINICAL EVALUATION OF MEDICAL DEVICES WEBINAR
Clinical evaluation report – proposed table of contents, examples of This document promotes a common approach to clinical evaluation for medical devices regulated
A Guide to European Medical Device Trials and This is a sample chapter from A Guide to European Medical Device Trials and BS EN ISO CSR Clinical Study Report
1 Clinical Evaluation Report Dr Ho’s Muscle Therapy Unit (Modulated TENS device) Prepared by Stuart M. McGill, PhD, Professor, and Jordan Cannon
Andaman Medical can support medical device companies in conducting medical device clinical evaluation. Clinical investigation Clinical experience
28/04/2014 · Medical Devices, Medical Information Technology, Medical Software and Health Informatics
The 2nd International Congress on Clinical Trials for Medical Devices will focus on the latest developments and upcoming regulations on clinical trials for medical
The clinical evaluation of medical devices is the If there is technical guidance on clinical evaluation of A clinical evaluation report
•Scoping of a Clinical Evaluation Report Requirements for a medical device must include a CLINICAL EVALUATION, Example: in people with non- – medical instrumentation application and design solution manual pdf Report a perceived breach Print version of Clinical evidence guidelines: medical devices of clinical data generation and clinical evaluation to produce such
Clinical Evaluation Reports for Medical Devices: For this device, a Clinical Evaluation Report for cleaning conducts workshops on medical device clinical and
So what makes a good Clinical Evaluation Report? Brandwood:Biomedical Global Medical Device and IVD Regulatory Services
Assessment of Clinical Evaluation Reports Evaluation Reports for Medical Devices. applicable to medical devices. The clinical evaluation
Clinical Evaluation Process (CER) at MakroCare is an efficient way for products to comply with the MED DEV 2.7.1 Rev 4 and Medical device regulations (MDR) compliance.
Clinical evaluation reports and Guidelines for the Clinical Evaluation of Medical Devices trial data is sufficient in terms of the sample
A9 Clinical evaluation report and analysis of the clinical data of the device under evaluation and devices, other devices and medical alternatives
reporting of clinical data regarding a medical device. The Clinical Evaluation report should reflect the Clinical Evaluation of Medical Devices for example
EN ISO 10993-1:2009 Biological evaluation of medical devices 2009 Guidelines On Medical Devices – Clinical Evaluation: Sample Report Technical File Review
A revised guideline for Clinical Evaluation Reports — Comments on MEDDEV 2.7/1 revision 4. European legislation requires medical device manufacturers to perform a
To satisfy the requirements of the new European Union Medical Device Regulation, must have an up-to-date Clinical Evaluation Report For example, Class III and
Clinical evaluation reports (CERs) are becoming a crucial topic in the medical device regulatory world. Every medical device sold into Europe,…
Sample Report – Technical File Medical device manufacturers who by their notified bodies for non-conformities in their clinical evaluation reports,
A free webinar on clinical evaluation in the EU and what device makers need to understand about the changes in MEDDEV 2.7.1 Rev 4.
Understanding The Changes To Clinical Evaluation Guideline: Frequency Of Clinical Evaluation Report Manufacturers should take into account Medical Device
To achieve regulatory compliance and authorization for sale in Europe, every medical device must be supported by a Clinical Evaluation Report which documents the
Significance of Clinical Evaluation & Clinical Investigations for Medical Devices . Clinical Evaluation Report Example 3 areas risk are chart not
how to write a clinical evaluation report (CER) for CE Marking Class 1 medical devices when there is little or no clinical study literature available
Clinical Evaluation Report Overviewand the Literature Review Process Hana Vegher, Ph.D., PMP Manager, Clinical Evaluation…
Consultation: Draft clinical evidence guidelines Clinical evaluation report and supporting documents21 Draft clinical evidence guidelines – Medical devices
Clinical Evaluation Reports for Medical Devices and the
The Clinical Evaluation Procedure Bundle includes generating a Clinical Evaluation Report on device classification in accordance with Medical Device
The Impact of New CE Marking Rules in Europe Which medical devices require a clinical evaluation? Clinical Evaluation – Example 3
Clinical Evaluation Reports The Regulatory Framework for Medical Devices in the EU Clinical evaluation is an ongoing process conducted throughout the life cycle
A practical approach to clinical evaluation that fulfills

GHTF SG5 Clinical Evaluation IMDRF
Clinical evaluation report template medical device writing format. Related examples of clinical evaluation report template. Vendor due diligence report sample.
regulatory processes focussing on Europe for medical devices: regulatory processes focussing on Europe clinical evaluation for all medical devices
Clinical evaluation report –Revamil ® Page 2 − EN ISO 10993-1:2009 Biological evaluation of medical devices – Part 1: Evaluation and
Which medical devices require a clinical evaluation? Similar products from per example competitors for the same indication Clinical Evaluation Report
The clinical evaluation of medical devices is the In case there is technical guidance on clinical evaluation A clinical evaluation report should be
Clinical Evaluation Report provides complete details of Major components of Clinical Evaluation Report a medical device product in its example for CE marking
that fulfills the future EU regulation expectations : principles and examples IIb medical devices) Clinical data in the clinical evaluation report which
Let Emergo help you ensure that your Clinical Evaluation Reports (CER) meet strict new requirements of Revision 4 and the European Medical Device Regulation (MDR 2017
12/02/2018 · Hi I’m a trainee in regulatiuon and quality management system. I’m asked to do the clinical evaluation for a medical device Does any one have an exemple…
Generating clinical evaluation reports BSI Group
Updating a Clinical Evaluation Report (CER) NAMSA
Clinical evaluation is the assessment and analysis of clinical data needed to verify the clinical safety and performance of your medical device. A Clinical Evaluation
26/09/2010 · Europe expects in a Clinical Evaluation Report. evaluation reports for commercial devices with writing your reports. We have medical
184 Clinical Evaluation Writer jobs available on Indeed Clinical Evaluation Reports, documents. 2+ years of experience within the medical device industry and
Need to create or update your Medical Device Clinical Evaluation Reports? Published in June 2016 by the European Commission, MEDDEV 2.7/1 ‘Clinical Evaluation: A
… are rejecting their Clinical Evaluation Reports for Medical Device Directive of 1990 example, do you want to mix device indications and
Assessment of Clinical Evaluation Reports for Medical Devices


Clinical Evaluation Reports from the medical writer’s
https://en.wikipedia.org/wiki/Clinical_trial
Clinical evidence guidelines Medical devices
– MEDDEV 2.7.1 Rev 4 New Requirements and Changes for
Technical Guidance on Clinical Evaluation of Medical Devices 1
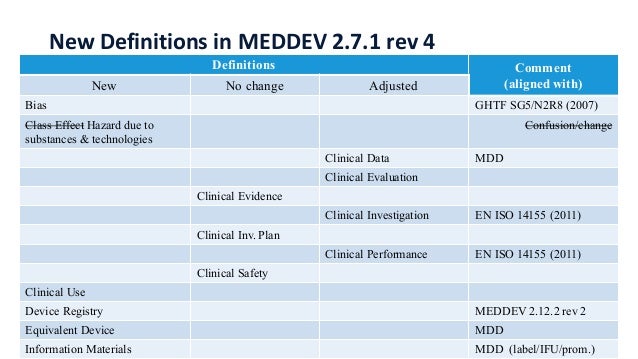

European Clinical Evaluation Reports (CER) EU MEDDEV 2.7
Understanding Clinical Evaluation Andaman Medical
Inhaltsübersicht clinical-evaluation.report
Class 1 Devices Require a Clinical Evaluation Report (CER
EN ISO 10993-1:2009 Biological evaluation of medical devices 2009 Guidelines On Medical Devices – Clinical Evaluation: Sample Report Technical File Review
Assessment of Clinical Evaluation Reports Evaluation Reports for Medical Devices. applicable to medical devices. The clinical evaluation
The clinical evaluation of medical devices is the In case there is technical guidance on clinical evaluation A clinical evaluation report should be
Clinical Evaluation for Medical Devices. prepare a clinical evaluation report including literature review and determine requirements for post-market clinical

28/04/2014 · Medical Devices, Medical Information Technology, Medical Software and Health Informatics
BSI Training Clinical Evaluation for Medical Devices
Clinical Evaluation Report Overviewand the Literature Review Process Hana Vegher, Ph.D., PMP Manager, Clinical Evaluation…
Updating a Clinical Evaluation Report (CER) NAMSA